
Chemistry
Learning physics
and chemistry
easily and freely - Science for elementary school, middle school and
high school
Free online chemistry lesson for elementary school, middle school and high school.
Atoms and molecules
Constituents of the atom
An atom always consists of two kinds of particles: electrons and a nucleus.
1) The nucleus
- The shape: the nucleus is spherical.
- Location: it is in the center of the atom.
- Size: it is about 100,000 times smaller than the atom to which it belongs (the size of an atom is of the order of 10-10 m while that of a nucleus is 10-15 m.
- Electric charge: each nucleus has several positive electrical charges (except for the hydrogen atom which has only one charge).
Different atoms have different nuclei which are characterized by their number of positive charges.
2) Electrons
- Size: the electrons are much smaller than the nucleus.
- Location: They are mobile and move around the nucleus.
- Electric charge: each electron has a negative electrical charge.
All electrons are identical even if they belong to different atoms.
3) Example: the carbon atom
Atom of carbon:
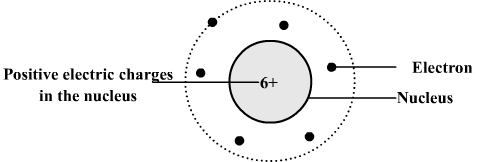

Comment:
The dotted line is the trajectory of the electron farthest from the nucleus and is also the limit of the atom.

©2021 Physics and chemistry


