
Chemistry
Learning physics
and chemistry
easily and freely - Science for elementary school, middle school and
high school
Free online chemistry lesson for elementary school, middle school and high school.
Water
Test for water
The essential
about test for water
How to identify water in a substance ?
This question worth asking because a liquid doesn't necessarily contain water, and a solid substance may contains water wich is not observable with the naked eye.
A Test for water must be used identify water.
Different tests exist but the most commonly used requires anhydrous copper sulphate.
Anhydrous copper sulphate
The test for water requires a chemical compound: Copper sulfate anhydrous. This is a white powder. Comment: The term anhydrous is formed of "an" (privative prefix) and "hydrous" meaning "water": "anhydrous" is therefore almost synonymous with dehydrated. |
 Photo: anhydrous copper sulphate
|
Copper sulphate formula
The complete name is copper II sulphate which indicates that it contains copper II ions ( whose formula is Cu2+ ) and Sulphate ions ( whose formula is SO42-).
Copper sulphate formula is therefore CuSO4.
Hydrated copper sulphate
Diagram: water and anhydrous
sulfate
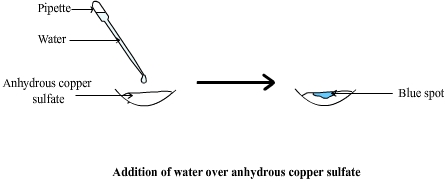
White anydrous copper sulphate that has been in contact with water becomes hydrated copper sulphate and turns blue. A copper sulfate hydrate still contains some CuSO4 cristals but also five water molecules ( H2O ) Copper sulfate hydrate can therefore also be called copper sulphate pentahydrate ( the penta prefix means "five" ). This change of color is exploited to test for water. |
 Photo: blue hydrated
copper sulphate and white anhydrous copper sulphate
|
Chemical test for water with anhydrous copper sulphate
Diagram: test for water in a
liquid
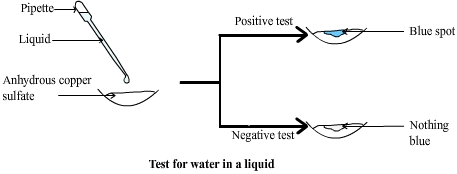
Diagram: test for water in a
solid
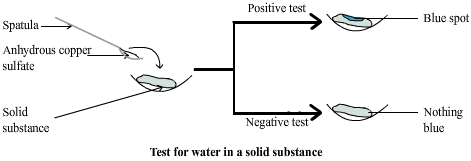

In each case if the copper sulphate turns blue it can be concluded that the substance contains water.
Some results obtained with the test for water
| Substance | Apple juice | Lemonade | Cola | Washing up liquid |
| Result | Positive | Positive | Positive | Negative |
| Is there water in the substance ? | Yes | Yes | Yes | No |
| Substance | Oil | Vinegar | Petroleum | Milk |
| Result | Negative | Positive | Negative | Positive |
| Is there water in the substance ? | No | Yes | No | Yes |
| Substance | Apple | Potato | Tomato | Paper |
| Result | Positive | Positive | Positive | Negative |
| Is there water in the substance ? | Yes | Yes | Yes | No |
Conclusion:
Most of the foods (liquid or solid) contain water but in different percentages:
Meat: about 60%
A potato: about 80%
An apple: 85%
A Potato: 93%
A salad: about 95%
A tomato: 98%
Note: fats (such as oil or butter) are exceptions because they do not contain water
Learn more about test for water
- Investigating the action of heat on copper sulphate: method to obtain anhydrous copper sulphate with hydrated copper sulphate.
- Hydrating anhydrous copper sulphate: video showing drops of water cast upon anhydrous copper sulphate.

©2021 Physics and chemistry


